Neuromodulation
Neuromodulation uses mild electrical stimulation of the sacral nerve. For patients with extreme frequency/urgency and who have failed other therapies, this therapy is a fairly new treatment option. Patients with urinary and/or fecal incontinence have also had positive results.
(A) Post Tibial Nerve Stimulation

First developed by Dr. Marshall Stoller at UCSF, Post Tibial Nerve Stimulation is the least invasive and most affordable neuromodulation option for patients to consider. During an office visit, a simple acupuncture needle is placed in a specific location about the ankle bone. It is then attached to a electrical stimulator and gently stimulates the post tibial nerve. It typically requires ten weekly treatments to determine if the procedure is beneficial. It is medicare approved and the most affordable neuromodulation option available due to the fact that it requires no multiple procedures and surgeries.
The Urgent PC Neuromodulation System by Uroplasty, Inc. is now available throughout the USA and abroad. There have been no serious adverse events filed with the FDA for this therapy. Only two mild reports are present in the FDA’s MAUDE database. A large number of published research and studies are available for your review on the Urgent PC website.
(B) Sacral Neuromodulation
Developed by Dr. Richard Schmidt and colleague, sacral neuromodulation is a more invasive form of therapy, requiring a trial procedure, implantation surgery, long-term management, programming and eventual revision, battery replacement and/or explantation surgery.
Patients are first required to undergo a test stimulation (an outpatient procedure which implants the electrode), which is a three to five day trial period of stimulation. If the results are favorable, doctors may then recommend a permanent implant. During the trial, patients will be asked to keep a voiding diary to track their symptoms. If, after that period, your diary indicates that it significantly helped your symptoms, you may be recommended to have the permanent implant.
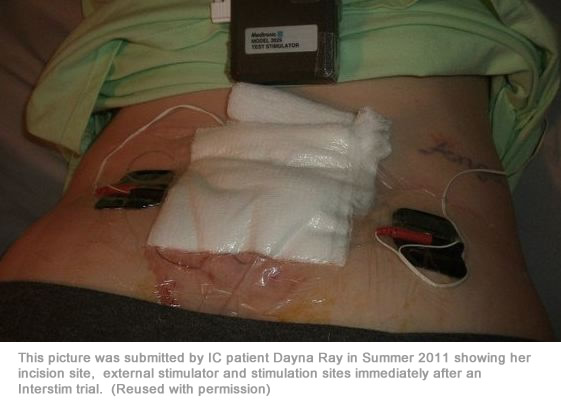
Interstim is approved for the treatment of OAB and it’s very easy for patients to get excited about this procedure as “the” long awaited answer to their symptoms. It’s VERY important that you take time to thoroughly research this before agreeing to have the procedure done. In our support forum, you’ll find patients on this web site who have had disappointing and, in some cases, terrible experiences with the procedure. You will also find patients who have found great results… usually in reducing their frequency.
 We urge caution when approaching this, the most controversial treatment approach, for IC. In the Summer of 2011, we discovered more than a twenty fatality reports that had been filed as adverse events relating, in some way, to Interstim. The FDA website offers no explanation as to why these deaths have occurred. We feel that further investigation is warranted and urge caution when exploring this therapy further. You can read these adverse event reports yourself by searching the FDA Manufacturer and User Device Experience (MAUDE) database.
We urge caution when approaching this, the most controversial treatment approach, for IC. In the Summer of 2011, we discovered more than a twenty fatality reports that had been filed as adverse events relating, in some way, to Interstim. The FDA website offers no explanation as to why these deaths have occurred. We feel that further investigation is warranted and urge caution when exploring this therapy further. You can read these adverse event reports yourself by searching the FDA Manufacturer and User Device Experience (MAUDE) database.
