67% of Patients Struggle to Pay For the Only Oral Drug Approved for IC/BPS by the US FDA
When the patent expired on Elmiron® in January 2010, the IC patient community expected the rapid release of a more affordable generic medication. Swati Spentose, a company located in India, has produced a generic pentosan polysulfate (Brand Name: Comfora®) currently available in a variety of countries, including Canada, but NOT in the USA. Why? They have yet to complete the rigorous FDA review required of all proposed generic medications. Unfortunately, that leaves patients in America prisoner to the high cost of this brand name medication. If not covered by insurance, some patients have been forced to pay more than $1500 per prescription for a medication which, ten years ago, cost $200 or less. The high economic toll of Elmiron® is undeniable.
Some patients have been forced to pay more than $1500 per prescription for a medication which, ten years ago, cost $200 or less.
In 2014, we noticed two new trends. Medicare and other health insurance programs were only covering the cost of Elmiron® for the first few months of the year. When patients hit the “donut hole” they were told that they would have to pay full price for this medication for the rest of the year. For a patient on disability or limited retirement bene- fits, this was impossible to do. Still other patients reported that their health insurance companies removed Elmiron® from their drug formularies with neither notice nor explanation.
ICN Elmiron® Patient Survey Results
In January 2015, we decided to probe the pricing and health insurance coverage changes for Elmiron® in an online survey. Completed by 923 IC patients who had been prescribed the medication and/or currently were taking it, the results demonstrate the economic toll that this medication is now taking. 17.9% said that the can barely afford it and have had to cut back substantially on other daily expenses to pay for their prescription. Another 17.7% said that paying for Elmiron® is challenging and that they’ve had to go without other essentials. 32.3% stated that they can pay for it but that it is a struggle. Only 32.2% said that they could afford it comfortably.
Patients also report a wide diversity in health insurance coverage, out of pocket costs and medication copayments. 6.5% of patients report- ed that Elmiron® was not covered by their health insurance while another 29.4% reported that it was only partially covered and that they had to pay the full price for at east six months of 2014. 61.1% reported that the medication was fully covered by their health insurance and that they only paid a small monthly copay. 3% said that they didn’t know if it was covered by their insurance.
Ultimately, 18% of our survey respondents chose to stop taking Elmiron® because they could no longer afford it.
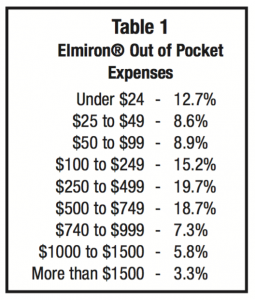 If not covered by insurance during 2014, 34% of patients paid more than $500 a month with 9.1% paying more than $1000 per month (see Table 1).
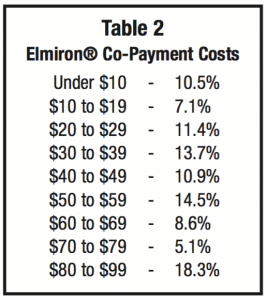
If not covered by insurance during 2014, 34% of patients paid more than $500 a month with 9.1% paying more than $1000 per month (see Table 1).If the medication was covered by insurance, copayments were evenly ranged from under $10 to $80-99. Most patients in this survey paid $80 or more per prescription (see Table 2).
There is a modest trend in health insurance cancellation of Elmiron® coverage. 18% of respondents reported that their Elmiron® coverage had been cancelled in recent years, with 3% in 2014. 1.5% reported cancellation between 2010 and 2013. Another 14% couldn’t remember when it was cancelled. On the other hand, 82% shared that it was cov- ered by their insurance to some degree.
Patients appear reluctant to talk about their financial hardships with their physicians. Only 32% shared their concern about the cost with their physicians. 7.4% of physicians suggested that patients reduce their dosage to help the prescription last longer. 7.4% suggested the use of other medications and/or OTC supplements. 17% of doctors had no suggestions to offer. Ultimately, 18% of our survey respondents chose to stop taking Elmiron® because they could no longer afford it.
 Despite the concerns about cost, patient satisfaction with Elmiron® was solid. 12.7% of patients felt that Elmiron® offered an excellent improvement in their symptoms and that their symptoms resolved almost completely with the treatment. 34% said that they had good improvement and that their symptoms reduced dramatically. They were grateful for its help. 17.2% said that they had a moderate improvement in their symptoms and that, with Elmiron®, they were able to function better in their daily life. and 14.5% noted only a slight improvement and that their IC still kept them from doing many things. 21.7% reported that they did not notice any improvement in their symptoms with Elmiron® use.
Despite the concerns about cost, patient satisfaction with Elmiron® was solid. 12.7% of patients felt that Elmiron® offered an excellent improvement in their symptoms and that their symptoms resolved almost completely with the treatment. 34% said that they had good improvement and that their symptoms reduced dramatically. They were grateful for its help. 17.2% said that they had a moderate improvement in their symptoms and that, with Elmiron®, they were able to function better in their daily life. and 14.5% noted only a slight improvement and that their IC still kept them from doing many things. 21.7% reported that they did not notice any improvement in their symptoms with Elmiron® use.
Most survey participants have taken Elmiron® for a meaningful period of time. 50% have taken Elmiron® for three or more years. 12% for two years, 14% for one year, 10% for six months, 9% for three months and only 5% took the med- ication less than a month.
Side Effects
Our survey also probed about the incidence of side effects. 36% reported no side effects while 64% reported a variety of effects including:
- mild hair loss (30%)
- headache (16%)
- minor diarrhea/loose bowels (15%)
- mild stomach upset/nausea (14%)
- dizziness (13%)
- depressed mood (9%)
- moderate to severe stomach upset/ nausea (7%)
- moderate to severe diarrhea (6%)
- skin rash (4%).
Eventually, 21% of respondents chose to stop Elmiron® due to side effects.
When side effects first began to appear is of interest. 23% reported side effects within the first week. 35% within the first month, 18% within the first three months, 10% at six months into treatment, 14% one year into treatment. Eventually, 21% of respondents chose to stop Elmiron® due to side effects.
Cost of Generic Medications Also Skyrocketing
We do not, at this time, have a price point for Comfora® if or when it becomes available in the USA. We hope that the price will be under $100 per month though industry developments in recent months suggest that that might not occur. Generic medications have increased in price so dramatically that a US Senate Subcommittee convened a hearing in November 2014. Vermont Senator Bernie Sanders shared “The prices of more than 1,200 generic medications increased an average of 448 percent between July 2013 and July 2014.” Some generics have surged in price by more than 1,000% percent.1
Pharmacists share the outrage of their customers. Pharmacist Rob Frankil, a member of the National Community Pharmacists Association, testified that he was accused of price gouging when a heart medication rose from $15 to $120 for a 90 day supply. 75% of pharmacists partici- pating in an NCPA survey reported higher prices on more than 25 generic drugs, with the prices spiking by 600% to 2,000% in certain cases.
Not surprisingly, the pharmaceutical companies remained silent and refused to offer an explanation. Every pharmaceutical company representative invited to testify at the hearing refused to attend.
What’s driving the Elmiron® Pricing Strategy?
Elmiron® has been shuffled through a variety of companies in the past twenty years, including Baker Norton Pharmaceuticals Inc., Alza, and Ortho-McNeil Pharmaceuticals. It is currently held by Jannsen Pharmaceuticals Inc., part of the health care behemoth Johnson & Johnson. They, too, have not offered an explanation as to why Elmiron® prices have increased leaving patients to wonder if they are simply trying to squeeze out as much profit as they can until a generic becomes available.
Patient Options
Years ago, an IC patient called the ICN office about Elmiron®. She said that she had invested her entire life savings on this medication and had run out of money. I asked her “Did it work for you?” She said “No.” I asked “Then why did you keep it taking it and spend your money?” She responded “I didn’t know that I could do anything else.”
In 2015, there are many more treatment options that you can explore. The American Urology Association Guidelines for IC/BPS offer a six step treatment protocol for IC that works well for many patients. The AUA Guidelines also encourage patients and providers to stop therapies which are not providing significant relief, which answers the age old question “Should I be on Elmiron for the rest of my life?” The answer, of course, is No. If it’s not working for you or you cannot afford it, it’s time to try other therapies that may offer similar, if not better, relief.
Many have turned to OTC nutraceuticals as mentioned in Step One of the AUA Guidelines (i.e. Bladder Builder®, Bladder Rest®, CystoMend®, CystoProtek®, Cysto Renew®). All contain chondroitin sulfate which is believed to have a bladder coating effect, as well as other beneficial ingredients. Better yet, they cost less than $50 for one bottle. Some physicians have also encouraged patients to reduce their dosage of Elmiron® to stretch out prescriptions so that they last for a longer period of time.
Patient Assistance Available
Johnson & Johnson offers a patient assistance program that may help patients who meet their income requirements. You may be eligible if you do not have public or private drug coverage, you reside in the USA and are being treated by a US licensed health care provider. If you are single, your income limit is $23,540 or less. Families of 2 must make $31,860 or less. Larger families will have income adjusted appropriately. Learn more by calling them at: 1-800-652-6227 or visit their website at: www.jjpaf.org
The high cost of treatment for the uninsured and underinsured makes Elmiron® treatment a significant barrier.
Conclusion
Regardless of its effectiveness, the high cost of treatment for the uninsured and underinsured makes Elmiron® treatment a significant barrier. Simply put, most can’t afford a therapy that costs $100 a month out of pocket, much less a staggering $1000 per month. We can only hope that the company listen to the cries of patients desperate for relief.
References:
- Hoey D. Generic Drug Costs Rise – Congress Asks Why. The Hill Congressional Blog. November 18, 2014. http://thehill.com/blogs/congress- blog/healthcare/224420-generic-drug-costs- rise-congress-asks-why
- Mohney G. Generic Drug Price Sticker Shock Prompts Probe by Congress. ABC News. November 21, 2014. http://abcnews.go.com/Health/generic-drug- prices-skyrocketing-lawmakers- warn/story?id=27060992

